Stefan Ploner, M. Sc.
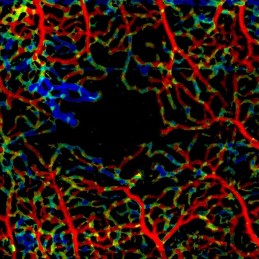
VISTA revealing slower blood flow in neovascularization (top-left of FAZ) in a motion-corrected OCTA scan of a 30 y/o PDR patient.
Measurement of blood flow speeds in retinal capillaries
In 2015, when OCT angiography was just being commercialized to map the structure of the retinal vasculature, I developed the first method for plexus-specific visualization of blood flow speed accross full-size enface OCT angiography images, Variable Interscan Time Analysis (VISTA) visualization [1]. The method was patented, orally presented at the ARVO annual meeting 2016 in front of an audience of ~1000 experts, and has attracted the attention of several research groups worldwide.
Volume Fusion and the Motion Correction and Volume Reconstruction in OCT framework (MoReOCT)
Especially OCT images from elderly subjects suffer from low SNR, as well as image artifacts like opacities, eye motion, and optical distortions. I developed a unique framework for automatic artifact correction and image enhancement. Multiple OCT scans are seamlessly fused within seconds, reaching unprecedented image qualities. Working together with collaboration partners and students, my method was central for the first in-vivo topograhpic mapping and quantification of basal laminar deposits [2], a potential early biomarker in age-related macular degeneration, the most widespread blinding eye disease among the elderly. Furthermore, the framework unvealed a subband below the external limiting membrane, which was not imaged in-vivo before, and allowed its association with age-related ocular alterations [3]. In addition, longitudinal data can be compared at the pixel scale, and features can be tracked across visits to investigate disease progression.
Collaborations with pioneering international experts
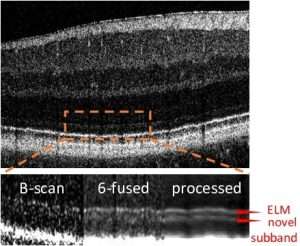
MoReOCT-based volume fusion unvealed a new retinal subband below the ELM.
I’m in close collaboration with the Biomedical Optical Imaging and Biophotonics Group at the Massachusetts Institute of Technology, the group who built the first and continues to develop most advanced ophthalmic OCT systems to date. Our joint developments are transferred to clinical application at the New England Eye Center, the first clinic that used OCT technology in patient care. I’m also studying new technologies with researchers at Oregon Health and Science University and Northwestern University.
My work is extensively evaluated on clinical data, was presented at international conferences like MICCAI, ISBI, ARVO and FLORetina [4-7], including multiple oral presentations, and was awarded with an Editor’s Pick from Biomedical Optics Express, a journal curated by leading OCT experts. Furthermore, I contributed a chapter on motion correction and volume fusion to the upcoming third edition of the book Optical Coherence Tomography.
Selected references:
[1] Retina, 2016. [2] IOVS, 2024. [3] TVST, 2025. [4] MICCAI, 2022. [5] ISBI, 2023. [6] ARVO, 2024. [7] ARVO, 2025.
Since 08/2017: PhD Student, Pattern Recognition Lab, Friedrich-Alexander University Erlangen-Nürnberg
- Research focus on motion correction and enhanced signal reconstruction in the field
of Optical Coherence Tomography (OCT) and OCT angiography (OCTA) - In collaboration with the Biomedical Optical Imaging and Biophotonics Group, MIT
- Published first-authored articles at MICCAI, ISBI and in Biomedical Optics Express
- Developed image processing methods that enabled clinical studies published in IOVS and TVST (1, 2)
- Journal reviewer for BOE, OE, IOVS, TVST, MedPhys, JBio, JBO, Optics Letters, and others
10/2014 – 05/2017: Master of Science with distinction in Computer Science, Friedrich-Alexander University Erlangen-Nürnberg
- Focus on medical image processing, pattern recognition and high-performance computing
- Master thesis: Improving 3D OCT motion correction (oral presentation at the ARVO annual meeting)
10/2015 – 05/2016: Visiting student, Massachusetts Institute of Technology, Cambridge, USA
- Member of the Biomedical Optical Imaging and Biophotonics Group under supervision of Prof. James Fujimoto
- Pioneering research in chorioretinal blood flow quantification based on VISTA-OCTA (oral presentation at the ARVO annual meeting)
04/2011 – 03/2015: Bachelor of Science in Computer Science, Friedrich-Alexander University Erlangen-Nürnberg
- Focus on pattern recognition, computer architecture, discrete optimization and computer graphics
- Student research assistant at the Pattern Recognition Lab, research on false
color visualization in the multispectral imaging software framework Gerbil
Teaching Experience
- 10/2014 – 01/2015: Student teaching assistant, Friedrich-Alexander University Erlangen-Nürnberg
- Exercises in Theory of Computation and Formal Languages
- 10/2011 – 09/2012: Student teaching assistant, Friedrich-Alexander University Erlangen-Nürnberg
- Exercises in Algorithms and Data Structures
- Editor’s pick in the journal Biomedical Optics Express (~5% of articles selected)
- ARVO international travel grant 2021
- Recognized as “exceptional reviewer” by the journals IOVS and TVST
- Graduation price from the German Physical Society for exceptional results in the high school final exam (Abitur)
- Motion correction & enhancement of volumetric high resolution OCT
FLORetina 2025, Fortezza da Basso, Florence, Italy - Advanced 3D image fusion for volumetric analysis of subtle features in retinal OCT
2024, Casey Eye Institute, Oregon Health and Science University, Portland, OR - A Spatiotemporal Illumination Model for 3D Image Fusion in Optical Coherence Tomography
IEEE International Symposium on Biomedical Imaging 2023, Cartagena, Colombia - A Spatiotemporal Model for Precise and Efficient Fully-automatic 3D Motion Correction in OCT
MICCAI main conference contribution presented at the Workshop on Biomedical Image Registration 2022, Singapore - 3-D OCT Motion Correction Efficiently Enhanced with OCT Angiography
ARVO annual meeting 2018, Honolulu, Hawaii - Toward quantitative OCT angiography: Visualizing flow impairment using variable interscan time analysis (VISTA)
ARVO annual meeting 2016, Ballroom A, B & C, Washington State Convention Center, Seattle, WA

2022
-
Temporally resolved 3-D retinal blood flow quantification using advanced motion correction and signal reconstruction in optical coherence tomography angiography
(Third Party Funds Single)
Project leader: ,
Term: since November 15, 2022
Acronym: 4D-OCTA
Funding source: DFG-Einzelförderung / Sachbeihilfe (EIN-SBH)Die optische Kohärenztomographie (OCT) erzeugt volumetrische 3-D-Bilder von Gewebe mit Mikrometerauflösung, indem sie einen Laserstrahl zum Scannen verwendet und die Amplitude und Zeitverzögerung von zurückgestreutem Licht misst. Die OCT hat einen großen Einfluss auf die Augenheilkunde und wurde zu einer Standard-Bildgebungsmodalität für die Diagnose, die Überwachung des Krankheitsverlaufs und das Ansprechen auf die Behandlung sowie für die Untersuchung der Pathogenese von Krankheiten wie diabetischer Retinopathie, altersbedingter Makuladegeneration und Glaukom. Die jüngste Entwicklung der OCT-Angiographie (OCTA) hat die grundlegende und klinische Forschung dramatisch beschleunigt. OCTA führt eine tiefenaufgelöste (3-D) Bildgebung der retinalen Mikrovaskulatur durch, indem es wiederholt die gleiche Netzhautposition abbildet und den Bewegungskontrast von sich bewegenden Blutzellen erkennt. Im Vergleich zu herkömmlichen Ansätzen, die auf injizierten Kontrastmitteln basieren, hat OCTA den Vorteil, dass es nicht invasiv ist, sodass die Bildgebung bei jedem Patientenbesuch durchgeführt werden kann, was Längsschnittstudien ermöglicht. Allerdings hat OCTA auch einige Einschränkungen. Da eine wiederholte Bildgebung erforderlich ist, um den Blutfluss zu erkennen, sind die Aufnahmezeiten lang und die Daten können durch Augenbewegungen und Bildartefakte verzerrt werden, was eine quantitative Längsschnittanalyse erschwert. OCTA-Algorithmen können das Vorhandensein eines Blutflusses erkennen, sind jedoch nur begrenzt in der Lage, subtile Veränderungen des Flusses aufzulösen, die frühe Anzeichen einer Krankheit sein können. Zeitliche Schwankungen des Flusses, die durch den Herzzyklus oder die funktionelle Reaktion der Netzhaut verursacht werden, sind schwer zu untersuchen. Wir schlagen vor, ein neues Framework für OCTA zu entwickeln, das eine Bewegungskorrektur auf Kapillarebene ermöglicht, Blutflussgeschwindigkeiten differenziert und eine Analyse auf mehreren Zeitskalen ermöglicht (4-D OCTA). Die Fähigkeit, über die Visualisierung der Mikrovaskulatur hinauszugehen und den Fluss und seine zeitlichen Schwankungen zu beurteilen, ermöglicht die Beurteilung subtiler Beeinträchtigungen der mikrovaskulären Perfusion sowie des Herzzyklus und der Reaktion auf funktionelle Stimulation. In Kombination mit der vaskulären strukturellen Bildgebung versprechen diese Fortschritte, neue Krankheitsmarker in früheren Krankheitsstadien bereitzustellen, eine genauere Messung des Krankheitsverlaufs und des Ansprechens auf die Therapie in pharmazeutischen Studien zu ermöglichen und zur Aufklärung der Pathogenese bei Netzhauterkrankungen beizutragen.
2017
-
Joint Iterative Reconstruction and Motion Compensation for Optical Coherence Tomography Angiography
(Third Party Funds Single)
Project leader: ,
Term: August 1, 2017 - July 31, 2019
Acronym: Joint Reco & MoCo for OCT(A)
Funding source: DFG-Einzelförderung / Sachbeihilfe (EIN-SBH)Optical coherence tomography (OCT) is a non-invasive 3-D optical imagingmodality that is a standard of care in ophthalmology [1,2]. Since the introduction of Fourier-domain OCT [3], dramatic increases in imaging speedbecame possible, enabling 3-D volumetric data to be acquired. Typically, aregion of the retina is scanned line by line, where each scanned lineacquires a cross-sectional image or a B-scan. Since B-scans are acquiredin milliseconds, slices extracted along a scan line, or the fast scanaxis, are barely affected by motion. In contrast, slices extractedorthogonally to scan lines, i. e. in slow scan direction, areaffected by various types of eye motion occurring throughout the full,multi-second volume acquisition time. The most relevant types of eyemovements during acquisition are (micro-)saccades, which can introducediscontinuities or gaps between B-scans, and slow drifts, which causesmall, slowly changing distortion [4]. Additional eye motion is caused by pulsatile blood flow,respiration and head motion. Despite ongoing advances in instrumentscanning speed [5,6] typical volume acquisition times havenot decreased. Instead, the additional scanning speed is used for densevolumetric scanning or wider fields of view [7]. OCT angiography (OCTA) [8–11] multiplies therequired number of scans by at least two, and even more scans are neededto accommodate recent developments in blood flow speed estimation whichare based on multiple interscan times [12,13]. As a consequence,there is an ongoing need for improvement in motion compensation especiallyin pathology [14–16].
We develop novel methods for retrospective motion correction of OCT volume scans of the anterior and posterior eye, and widefield imaging. Our algorithms are clinically usable due to their suitability for patients with limited fixation capabilities and increased amount of motion, due to their fast processing speed, and their high accuracy, both in terms of alignment and motion correction. By merging multiple accurately aligned scans, image quality can be increased substantially, enabling the inspection of novel features.
2025
Journal Articles
- , , , , , , , , , :
High-Resolution, Motion-Corrected, Volume-Fused OCT for Investigating Longitudinal Changes in Subretinal Drusenoid Deposits in Intermediate AMD
In: Translational Vision Science & Technology 14 (2025), Article No.: 15
ISSN: 2164-2591
DOI: 10.1167/tvst.14.6.15
BibTeX: Download - , , , , , , , , , , , , , , , :
High-Resolution OCT Reveals Age-Associated Variation in the Region Posterior to the External Limiting Membrane
In: Translational Vision Science & Technology 14 (2025), p. 16-
ISSN: 2164-2591
DOI: 10.1167/tvst.14.1.16
BibTeX: Download
2024
Journal Articles
- , , , , , , , :
Glaucoma detection using non-perfused areas in OCTA
In: Scientific Reports 14 (2024), Article No.: 10306
ISSN: 2045-2322
DOI: 10.1038/s41598-024-60839-4
BibTeX: Download - , , , , , , , , :
Ensembling U-Nets for microaneurysm segmentation in optical coherence tomography angiography in patients with diabetic retinopathy
In: Scientific Reports 14 (2024), Article No.: 21520
ISSN: 2045-2322
DOI: 10.1038/s41598-024-72375-2
URL: https://rdcu.be/dT0FK
BibTeX: Download - , , , , , , , :
Impact of acquisition area on deep-learning-based glaucoma detection in different plexuses in OCTA
In: Scientific Reports 14 (2024), Article No.: 20414
ISSN: 2045-2322
DOI: 10.1038/s41598-024-71235-3
BibTeX: Download - , , , , , , , , , , , , , , , , :
Topographic Measurement of the Subretinal Pigment Epithelium Space in Normal Aging and Age-Related Macular Degeneration Using High-Resolution OCT
In: Investigative Ophthalmology & Visual Science 65 (2024), Article No.: 18
ISSN: 0146-0404
DOI: 10.1167/iovs.65.10.18
BibTeX: Download
Conference Contributions
- , , , , , , , :
3D Deep Learning-based Boundary Regression of an Age-related Retinal Biomarker in High Resolution OCT
German Conference on Medical Image Computing, BVM 2024 (Erlangen, March 10, 2024 - March 12, 2024)
In: Andreas Maier, Thomas M. Deserno, Heinz Handels, Klaus Maier-Hein, Christoph Palm, Thomas Tolxdorff (ed.): Bildverarbeitung für die Medizin 2024. BVM 2024, Wiesbaden: 2024
DOI: 10.1007/978-3-658-44037-4_90
BibTeX: Download - , , , , , , , , , , :
Age-Related Variations in the External Limiting Membrane and the Ellipsoid Zone
ARVO annual meeting (Seattle, WA, May 5, 2024 - May 9, 2024)
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2793996
URL: https://iovs.arvojournals.org/article.aspx?articleid=2793996
BibTeX: Download - , , , , , , , , , :
A reliable, fully-automatic pipeline for 3D motion correction and volume fusion enables investigation of smaller and lower-contrast OCT features
ARVO annual meeting 2024 (Seattle, WA, May 5, 2024 - May 9, 2024)
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2794904
URL: https://iovs.arvojournals.org/article.aspx?articleid=2794904
BibTeX: Download - , , , , , , , , , , , , , , :
Topographic measurement of the sub-retinal pigment epithelium space in normal aging and age-related macular degeneration using volumetric high-resolution OCT
ARVO imaging in the eye conference (Seattle, WA, May 4, 2024 - May 4, 2024)
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2800456
URL: https://iovs.arvojournals.org/article.aspx?articleid=2800456
BibTeX: Download - , , , , , , , , , , :
Volumetric High-Resolution Optical Coherence Tomography Reveals Longitudinal Changes in Subretinal Drusenoid Deposits in Intermediate Dry Age-related Macular Degeneration
ARVO annual meeting 2024 (Seattle, WA, May 5, 2024 - May 9, 2024)
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2795385
URL: https://iovs.arvojournals.org/article.aspx?articleid=2795385
BibTeX: Download
2023
Journal Articles
- , , , , , , , , , , , :
Retinal blood flow speed quantification at the capillary level using temporal autocorrelation fitting OCTA [Invited]
In: Biomedical Optics Express 14 (2023), p. 2658-2677
ISSN: 2156-7085
DOI: 10.1364/BOE.488103
BibTeX: Download
Conference Contributions
- , , , , , , , :
A Spatiotemporal Illumination Model for 3d Image Fusion in Optical Coherence Tomography
IEEE International Symposium on Biomedical Imaging (Cartagena, April 18, 2023 - April 21, 2023)
In: 2023 IEEE 20th International Symposium on Biomedical Imaging 2023
DOI: 10.1109/ISBI53787.2023.10230526
BibTeX: Download - , , , , , , , , , :
Advanced volume rebuilding overcomes quilting, stretching, and banding image artifacts in orthogonally-scanned OCT
Arvo Annual Meeting 2023 (New Orleans)
In: Investigative Ophthalmology & Visual Science 2023
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2789933
BibTeX: Download - , , , , , , :
Impact of acquisition area on deep-learning-based glaucoma detection in OCTA
ARVO annual meeting 2023 (New Orleans)
In: Investigative Ophthalmology & Visual Science 2023
BibTeX: Download - , , , , , , , :
Abstract: A Spatiotemporal Model for Precise and Efficient Fully-automatic 3D Motion Correction in OCT
Bildverarbeitung für die Medizin Workshop, BVM 2023 (Braunschweig, DEU, July 2, 2023 - July 4, 2023)
In: Thomas M. Deserno, Heinz Handels, Andreas Maier, Klaus Maier-Hein, Christoph Palm, Thomas Tolxdorff (ed.): Informatik aktuell 2023
DOI: 10.1007/978-3-658-41657-7_57
BibTeX: Download - , , , , , , , , , , , :
Motion correction and volume merging of ultrahigh resolution OCT enable 3D visualization and longitudinal tracking of hyperreflective foci
ARVO imaging in the eye (New Orleans, April 22, 2023 - April 22, 2023)
In: Investigative Ophthalmology & Visual Science 2023
URL: https://iovs.arvojournals.org/article.aspx?articleid=2791149
BibTeX: Download
2022
Journal Articles
- , , , , , , , , , :
Trainable joint bilateral filters for enhanced prediction stability in low-dose CT
In: Scientific Reports 12 (2022), Article No.: 17540
ISSN: 2045-2322
DOI: 10.1038/s41598-022-22530-4
BibTeX: Download - , , , , , , , , , , , :
Ultra low‐parameter denoising: Trainable bilateral filter layers in computed tomography
In: Medical Physics 49 (2022), p. 5107-5120
ISSN: 0094-2405
DOI: 10.1002/mp.15718
BibTeX: Download
Conference Contributions
- , , , , , , , :
A Spatiotemporal Model for Precise and Efficient Fully-Automatic 3D Motion Correction in OCT
25th International Conference on Medical Image Computing and Computer-Assisted Intervention, MICCAI 2022 (Singapore, SGP, September 18, 2022 - September 22, 2022)
In: Linwei Wang, Qi Dou, P. Thomas Fletcher, Stefanie Speidel, Shuo Li (ed.): Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 2022
DOI: 10.1007/978-3-031-16434-7_50
BibTeX: Download - , , , , , , , :
Ultrahigh resolution OCT, volume merging, and advanced signal reconstruction improve visualization of the RPE-Bruch's complex
In: Investigative Ophthalmology & Visual Science, Rockville: 2022
URL: https://iovs.arvojournals.org/article.aspx?articleid=2782224
BibTeX: Download - , , , , , , :
Unsupervised OCT Denoising using speckle split
ARVO annual meeting 2022 (Denver)
In: INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE, ROCKVILLE: 2022
DOI: 10.1038/s41598-023-37324-5
BibTeX: Download
2021
Journal Articles
- , , , , , , , , :
Glaucoma classification in 3 x 3 mm en face macular scans using deep learning in a different plexus
In: Biomedical Optics Express 12 (2021), p. 7434-7444
ISSN: 2156-7085
DOI: 10.1364/BOE.439991
URL: https://opg.optica.org/boe/fulltext.cfm?uri=boe-12-12-7434&id=464725
BibTeX: Download - , , , , , , , , , :
Efficient and high accuracy 3-D OCT angiography motion correction in pathology
In: Biomedical Optics Express 12 (2021), p. 125-146
ISSN: 2156-7085
DOI: 10.1364/BOE.411117
URL: https://www.osapublishing.org/boe/fulltext.cfm?uri=boe-12-1-125
BibTeX: Download - , , , , , , , , , :
Maximum a posteriori signal recovery for optical coherence tomography angiography image generation and denoising
In: Biomedical Optics Express 12 (2021), p. 55-68
ISSN: 2156-7085
DOI: 10.1364/BOE.408903
URL: https://www.osapublishing.org/boe/fulltext.cfm?uri=boe-12-1-55&id=444277
BibTeX: Download - , , , , , , , , , :
OCT-OCTA segmentation: Combining structural and blood flow information to segment bruch's membrane
In: Biomedical Optics Express 12 (2021), p. 84-99
ISSN: 2156-7085
DOI: 10.1364/BOE.398222
BibTeX: Download
Conference Contributions
- , , , , , , , , , :
Applying nnU-Net to the segmentation of microaneurysms in OCTA data in patients with non-proliferative diabetic retinopathy
ARVO Annual Meeting 2021
URL: https://iovs.arvojournals.org/article.aspx?articleid=2773716
BibTeX: Download - , , , , , , :
Deep Learning Glaucoma Classification in enface OCTA scans of the human macular region
ARVO Annual Meeting 2021
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2773575
URL: https://iovs.arvojournals.org/article.aspx?articleid=2773575
BibTeX: Download - , , , , , , , :
Improved OCT motion correction in pathology using advanced eye motion modeling
In: INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE, ROCKVILLE: 2021
BibTeX: Download - , , , , , , , , , :
Abstract: Maximum A-posteriori Signal Recovery for OCT Angiography Image Generation
German Workshop on Medical Image Computing, 2021 (Regensburg, March 7, 2021 - March 9, 2021)
In: Christoph Palm, Heinz Handels, Klaus Maier-Hein, Thomas M. Deserno, Andreas Maier, Thomas Tolxdorff (ed.): Informatik aktuell 2021
DOI: 10.1007/978-3-658-33198-6_61
BibTeX: Download
2020
Journal Articles
- , , , , , , , , , :
A Framework for Multiscale Quantitation of Relationships Between Choriocapillaris Flow Impairment and Geographic Atrophy Growth
In: American Journal of Ophthalmology (2020)
ISSN: 0002-9394
DOI: 10.1016/j.ajo.2019.12.006
BibTeX: Download
Conference Contributions
- , , , , , , , :
Ultrahigh-Resolution Optical Coherence Tomography Investigation of Age-Related Changes in the Photoreceptors, Retinal Pigment Epithelium and Bruch's Membrane
Annual Meeting of the Association-for-Research-in-Vision-and-Ophthalmology (ARVO) (Online, May 1, 2020 - May 7, 2020)
In: INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE, ROCKVILLE: 2020
BibTeX: Download - , , , , , , :
Fully automatic separation of three capillary plexuses in the macular region
Annual Meeting of the Association-for-Research-in-Vision-and-Ophthalmology (ARVO) (ONLINE, May 1, 2020 - May 7, 2020)
In: INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE, ROCKVILLE: 2020
BibTeX: Download - , , , , , , , :
Investigating Subtle Structural Changes in Eyes with Age-Related Macular Degeneration: A Pilot Study Using Ultrahigh-Resolution Optical Coherence Tomography
Annual Meeting of the Association-for-Research-in-Vision-and-Ophthalmology (ARVO) (ONLINE, May 1, 2020 - May 7, 2020)
In: INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE, ROCKVILLE: 2020
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2769686
URL: https://iovs.arvojournals.org/article.aspx?articleid=2769686
BibTeX: Download - , , , , , , :
3-D tracing of capillaries through three retinal vascular plexuses toward their closest superficial artery and vein in OCT angiography volumes
ARVO annual meeting 2020
In: Investigative Ophthalmology & Visual Science 2020
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2770859
URL: https://iovs.arvojournals.org/article.aspx?articleid=2770859
BibTeX: Download - , , , , , , :
Compressed sensing for optical coherence tomography angiography volume generation
International workshop on Algorithmen - Systeme - Anwendungen, 2020 (Berlin, March 15, 2020 - March 17, 2020)
In: Thomas Tolxdorff, Thomas M. Deserno, Heinz Handels, Andreas Maier, Klaus H. Maier-Hein, Christoph Palm (ed.): Informatik aktuell 2020
DOI: 10.1007/978-3-658-29267-6_19
BibTeX: Download - , , , , :
Modularization of deep networks allows cross-modality reuse: lesson learnt
International workshop on Algorithmen - Systeme - Anwendungen, 2020 (Berlin, March 15, 2020 - March 17, 2020)
In: Thomas Tolxdorff, Thomas M. Deserno, Heinz Handels, Andreas Maier, Klaus H. Maier-Hein, Christoph Palm (ed.): Informatik aktuell 2020
DOI: 10.1007/978-3-658-29267-6_61
BibTeX: Download - , , , , :
Open source simulation of fixational eye drift motion in oct scans: Towards better comparability and accuracy in retrospective OCT motion correction
International workshop on Algorithmen - Systeme - Anwendungen, 2020 (Berlin, March 15, 2020 - March 17, 2020)
In: Thomas Tolxdorff, Thomas M. Deserno, Heinz Handels, Andreas Maier, Klaus H. Maier-Hein, Christoph Palm (ed.): Informatik aktuell 2020
DOI: 10.1007/978-3-658-29267-6_56
BibTeX: Download
2019
Journal Articles
- , , , , , :
Technical Note: PYRO-NN: Python reconstruction operators in neural networks
In: Medical Physics 46 (2019), p. 5110-5115
ISSN: 0094-2405
DOI: 10.1002/mp.13753
URL: https://aapm.onlinelibrary.wiley.com/doi/abs/10.1002/mp.13753
BibTeX: Download - , , , , , , , , , , , , , :
Spatial Distribution of Choriocapillaris Impairment in Eyes with Choroidal Neovascularization Secondary to Age-Related Macular Degeneration
In: Retina-The Journal of Retinal and Vitreous Diseases (2019)
ISSN: 0275-004X
DOI: 10.1097/IAE.0000000000002556
URL: https://journals.lww.com/retinajournal/Abstract/publishahead/SPATIAL_DISTRIBUTION_OF_CHORIOCAPILLARIS.96058.aspx
BibTeX: Download
Conference Contributions
- , , , , , , , , :
Correction of artifacts from misregistered B-scans in orthogonally scanned and registered OCT angiography
ARVO Annual Meeting 2019 (Vancouver, B.C., Canada, April 28, 2019 - May 2, 2019)
In: Investigative Ophthalmology & Visual Science 2019
URL: https://iovs.arvojournals.org/article.aspx?articleid=2745781&resultClick=1
BibTeX: Download - , , , , , , :
Deep learning based hybrid OCT-OCTA segmentation of Bruch's membrane in pathology
Annual Meeting of the Association-for-Research-in-Vision-and-Ophthalmology (ARVO) (Vancouver, April 28, 2019 - May 2, 2019)
In: INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE, ROCKVILLE: 2019
BibTeX: Download - , , , , , , , :
Relationship between CC Impairment and GA Growth: An Optical Coherence Tomography Angiography Study
Annual Meeting of the Association-for-Research-in-Vision-and-Ophthalmology (ARVO) (Vancouver, April 28, 2019 - May 2, 2019)
In: INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE, ROCKVILLE: 2019
BibTeX: Download - , , , , , , , , :
Using Medical Image Reconstruction Methods for Denoising of OCTA Data
ARVO Annual Meeting 2019 (Vancouver, B.C., Canada, April 28, 2019 - May 2, 2019)
In: Investigative Ophthalmology & Visual Science 2019
URL: https://iovs.arvojournals.org/article.aspx?articleid=2746928&resultClick=1
BibTeX: Download - , , , , :
Respiratory Deformation Estimation in X-Ray-Guided IMRT Using a Bilinear Model
Workshop on Bildverarbeitung fur die Medizin, 2019 (Lübeck, DEU, March 17, 2019 - March 19, 2019)
In: Thomas M. Deserno, Andreas Maier, Christoph Palm, Heinz Handels, Klaus H. Maier-Hein, Thomas Tolxdorff (ed.): Informatik aktuell 2019
DOI: 10.1007/978-3-658-25326-4_70
BibTeX: Download
2018
Journal Articles
- , , , , , , , , , , , , , , , , , :
Analyzing relative blood flow speeds in choroidal neovascularization using variable interscan time analysis OCT angiography
In: Ophthalmology Retina 2 (2018), p. 306-319
ISSN: 2468-7219
DOI: 10.1016/j.oret.2017.08.013
URL: https://www.sciencedirect.com/science/article/abs/pii/S2468653017303561?via=ihub
BibTeX: Download
Book Contributions
- , , :
Optical Coherence Tomography
In: Andreas MaierStefan SteidlVincent ChristleinJoachim Hornegger (ed.): Medical Imaging Systems, Cham: Springer, 2018, p. 251-261 (Lecture Notes in Computer Science book series (LNCS), Vol.11111)
ISBN: 978-3-319-96520-8
DOI: 10.1007/978-3-319-96520-8_12
URL: https://link.springer.com/chapter/10.1007/978-3-319-96520-8_12
BibTeX: Download
Conference Contributions
- , , , , , , :
A Joint Probabilistic Model for Speckle Variance, Amplitude Decorrelation and Interframe Variance (IFV) Optical Coherence Tomography Angiography
Bildverarbeitung für die Medizin 2018 - Algorithmen - Systeme - Anwendungen (Erlangen, March 11, 2018 - March 13, 2018)
In: Proceedings des Workshops Bildverarbeitung für die Medizin 2018 - Algorithmen - Systeme - Anwendungen 2018
DOI: 10.1007/978-3-662-56537-7_37
BibTeX: Download - , , , , , , , , , , , , , , :
OCT-OCTA Segmentation
Bildverarbeitung für die Medizin 2018 (Erlangen, March 11, 2018 - March 13, 2018)
In: Maier, Andreas Deserno, Thomas Martin Handels, Heinz Maier-Hein, Klaus C., Palm Tolxdorff, Thomas (ed.): Bildverarbeitung für die Medizin 2018, Berlin: 2018
DOI: 10.1007/978-3-662-56537-7_76
URL: https://link.springer.com/chapter/10.1007/978-3-662-56537-7_76
BibTeX: Download - , , , , , , , , , , , , , :
3-D OCT Motion Correction Efficiently Enhanced with OCT Angiography
ARVO annual meeting 2018
In: Investigative Ophthalmology & Visual Science 2018
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2694583
URL: https://iovs.arvojournals.org/article.aspx?articleid=2694583
BibTeX: Download
2017
Journal Articles
- , , , , , , , , , , , , , :
The Definition, Rationale, and Effects of Thresholding in OCT Angiography
In: Ophthalmology Retina 1/2017 (2017), p. 435-447
ISSN: 2468-7219
DOI: 10.1016/j.oret.2017.01.019
URL: https://www.sciencedirect.com/science/article/abs/pii/S2468653017300283
BibTeX: Download - , , , , , , , , , , , :
Polypoidal Choroidal Vasculopathy on Swept-Source Optical Coherence Tomography Angiography with Variable Interscan Time Analysis
In: Translational Vision Science & Technology 6 (2017)
ISSN: 2164-2591
DOI: 10.1167/tvst.6.6.4
URL: https://www5.informatik.uni-erlangen.de/Forschung/Publikationen/2017/Rebhun17-PCV.pdf
BibTeX: Download
Conference Contributions
- , , , , , , , , , , :
Hybrid OCT-OCTA Vessel Visualization for Projection-Free Display of the Intermediate and Deep Retinal Plexuses
ARVO Annual Meeting 2017 (Baltimore, MD, USA, May 7, 2017 - May 11, 2017)
In: Investigative Ophthalmology & Visual Science 2017
URL: https://www5.informatik.uni-erlangen.de/Forschung/Publikationen/2017/Ploner17-HOV.pdf
BibTeX: Download - , , , , , , , , , , , , , , :
OCT-OCTA Segmentation: a Novel Framework and an Application to Segment Bruch's Membrane in the Presence of Drusen
ARVO Annual Meeting 2017 (Baltimore, MD, USA, May 7, 2017 - May 11, 2017)
In: Investigative Ophthalmology & Visual Science 2017
URL: https://www5.informatik.uni-erlangen.de/Forschung/Publikationen/2017/Schottenhamml17-OSA.pdf
BibTeX: Download - , , , , , , , , , , , , :
Parallel Variable Interscan Time Analysis (VISTA) OCTA and en Face Doppler OCT of Optic Disc and Peripapillary Vasculature
ARVO Annual Meeting 2017 (Baltimore, MD, USA, May 7, 2017 - May 11, 2017)
In: Investigative Ophthalmology & Visual Science 2017
URL: https://www5.informatik.uni-erlangen.de/Forschung/Publikationen/2017/Lee17-PVI.pdf
BibTeX: Download - , , , , , , , , , :
Smoothed and Resolved Thresholding (SmaRT-) Display: A New OCTA Display Technique to Resolve the Low Flow Ambiguity
ARVO Annual Meeting 2017 (Baltimore, MD, USA, May 7, 2017 - May 11, 2017)
In: Investigative Ophthalmology & Visual Science 2017
URL: https://www5.informatik.uni-erlangen.de/Forschung/Publikationen/2017/Yiu17-SAR.pdf
BibTeX: Download - , , , , , , , , , , , , , :
Visualizing microvascular flow variation in OCTA using variable interscan time analysis (VISTA)
SPIE BiOS (San Francisco, CA, USA, January 28, 2017 - February 2, 2017)
In: Proc. SPIE 10053, Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XXI 2017
DOI: 10.1117/12.2254845
URL: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10053/1/Visualizing-microvascular-flow-variation-in-OCTA-using-variable-interscan-time/10.1117/12.2254845.full?SSO=1
BibTeX: Download - , , , , , , , , , , , , , :
Analysis of Polypoidal Choroidal Vasculopathy Using Swept Source Optical Coherence Tomography Angiography with Variable Interscan Time Analysis
ARVO Annual Meeting 2017 (Baltimore, MD, USA)
In: Investigative Ophthalmology & Visual Science, ARVO: 2017
URL: https://www5.informatik.uni-erlangen.de/Forschung/Publikationen/2017/Moreira-Neto17-AOP.pdf
BibTeX: Download - , , , , , , , , , , , , :
Correlation of Vascular Impairment with Geographic Atrophy Progression Analyzed with Swept Source OCT Angiography and Variable Interscan Time Analysis
ARVO annual meeting 2017 (Baltimore)
BibTeX: Download - , , , , , , , , , , , , , :
Variable Interscan Time Analysis (VISTA-) Optical Coherence Tomography Angiography (OCTA) of Blood Flow Speeds in Eyes with Diabetic Retinopathy
ARVO Annual Meeting 2017 (Baltimore, MD, USA, May 7, 2017 - May 11, 2017)
In: Investigative Ophthalmology & Visual Science, ARVO: 2017
URL: https://iovs.arvojournals.org/article.aspx?articleid=2637998&resultClick=1
BibTeX: Download - , , , , , , , , , , , , :
Visualizing Relative Blood Flow Speeds in Choroidal Neovascularization Using Variable Interscan Time Analysis (VISTA-) Optical Coherence Tomography Angiography (OCTA)
ARVO Annual Meeting 2017 (Baltimore, MD, USA, May 7, 2017 - May 11, 2017)
In: Investigative Ophthalmology & Visual Science 2017
Open Access: https://iovs.arvojournals.org/article.aspx?articleid=2637740
URL: https://iovs.arvojournals.org/article.aspx?articleid=2637740
BibTeX: Download
2016
Journal Articles
- , , , , , , , , , , , , :
An Automatic, Intercapillary Area-based Algorithm for Quantifying Diabetes-related Capillary Dropout Using Optical Coherence Tomography Angiography
In: Retina (Philadelphia, Pa.) 36 (2016), p. S93-S101
ISSN: 1539-2864
DOI: 10.1097/IAE.0000000000001288
URL: https://www.ncbi.nlm.nih.gov/pubmed/28005667
BibTeX: Download - , , , , , , , , , , , :
Swept-source optical coherence tomography angiography reveals choriocapillaris alterations in eyes with nascent geographic atrophy and drusen-associated geographic atrophy
In: Retina-The Journal of Retinal and Vitreous Diseases 36 (2016), p. S2-S11
ISSN: 0275-004X
DOI: 10.1097/IAE.0000000000001287
URL: https://journals.lww.com/retinajournal/Fulltext/2016/12001/SWEPT_SOURCE_OPTICAL_COHERENCE_TOMOGRAPHY.2.aspx
BibTeX: Download - , , , , , , , , , , , , , , :
Toward Quantitative Optical Coherence Tomography Angiography: Visualizing Blood Flow Speeds in Ocular Pathology Using Variable Interscan Time Analysis
In: Retina (Philadelphia, Pa.) 32 (2016), p. S118-S126
ISSN: 1539-2864
DOI: 10.1097/IAE.0000000000001328
BibTeX: Download
Conference Contributions
- , , , , , , , , , :
An automatic algorithm measuring the retinal intercapillary area to assess diabetic retinopathy
ARVO Annual Meeting 2016 (Seattle, WA, USA, May 1, 2016 - May 5, 2016)
In: Investigative Ophthalmology & Visual Science 2016
URL: https://www5.informatik.uni-erlangen.de/Forschung/Publikationen/2016/Schottenhamml16-AAA.pdf
BibTeX: Download - , , , , , , , , , :
Toward quantitative OCT angiography: visualizing flow impairment using variable interscan time analysis (VISTA)
ARVO Annual Meeting 2016 (Seattle, WA, USA, May 1, 2016 - May 5, 2016)
In: Investigative Ophthalmology & Visual Science 2016
URL: https://www5.informatik.uni-erlangen.de/Forschung/Publikationen/2016/Ploner16-TQO.pdf
BibTeX: Download


