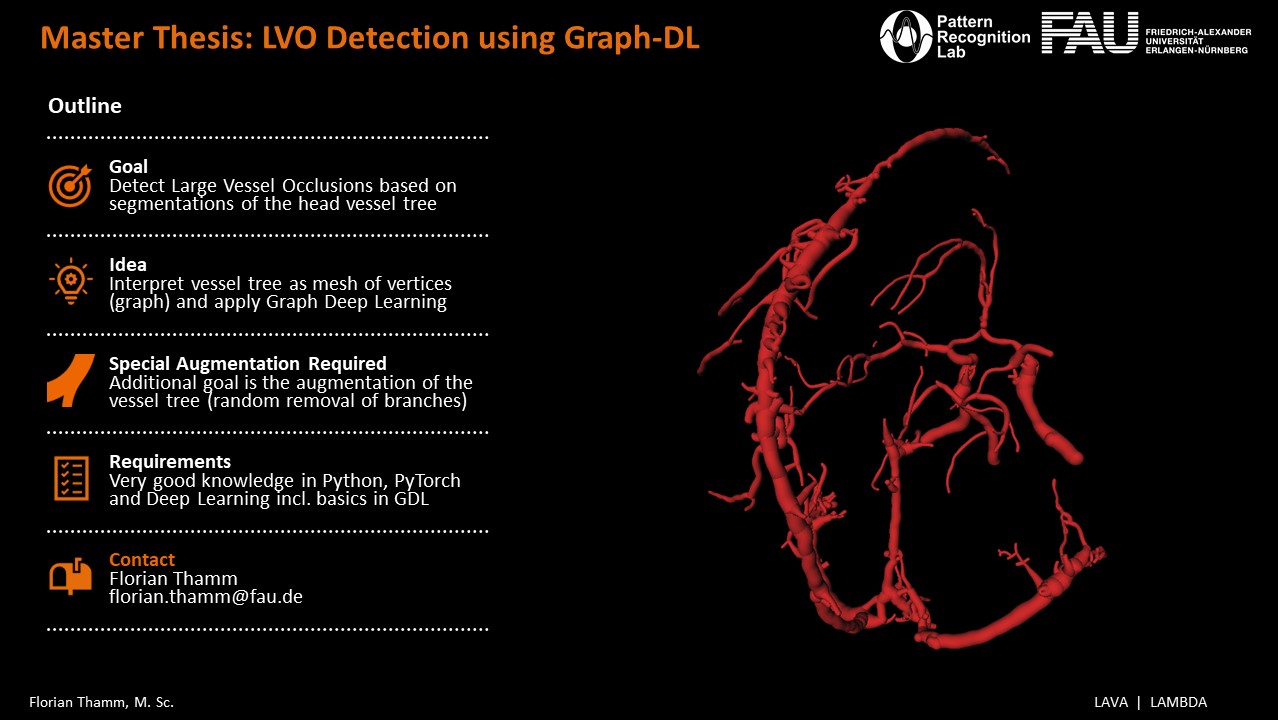
Thesis Description
Acute ischemic stroke (AIS) is among the leading causes of death (9%) and disability worldwide and will increase over the next 20 years [1, 2]. One of the main causes for an AIS is large vessel occlusion (LVO),accounting for more than one-third of all AIS cases [3]. Thus, making it a key component in the diagnostic process and treatment [4]. These occlusions usually occurs in the internal carotid artery, proximal middle cerebral artery or basilar artery. As a treatment for AIS thrombolysis has been used over the years in a cautious manner, due to the limited application time window and the need to asses the risks to benefits ratio individually. An alternative preferred treatment method is the mechanical thrombectomy, where patients are treated surgically [5, 6]. Thereby, doing a fast classification and possibly a detection of LVOs is crucial for a successful diagnosis and thus for the treatment of AIS [4, 6].
Computed Tomography Angiography (CTA) represents one of the most important techniques in order to detect LVOs leading to the diagnosis and treatment management of AIS, by visualizing the cerebrovascular vessel tree [7]. Detecting such LVOs manually is very time consuming, especially when handling big datasets. Hence an automated classification of patients suffering from LVOs is beneficial for the clinical workflow, by shortening the diagnostic time and improving on accurate classification. For this purpose multiple algorithms were implemented and tested for such application using simple machine learning methods, such as regularized logistic regression, linear support vector machines, and random forest, providing good results as presented by Nishi et al. in [8]. In the last couple of years more sophisticated methods like deep learning, in particular convolutional neural networks (CNNs) as seen in [9, 10] are applied and improve previous algorithms. Still traditional deep learning tends to fail to modulate irregular complex structures, such as cerebrovascular vessel trees. This is mainly due to the fact, that the topology differs between patients [11]. Thus, a plausible alternative should be tested.
Graph deep learning has emerged in recent years as a powerful tool in machine learning, using graphs to model geometrical and topological relationships. It provides an advantage over traditional deep learning regarding irregular structures, such as cerebrovascular vessel trees [11]. Another reason for cosidering GDL for detecting LVOs besides the deformations invariance is the dimension reduction of the inputdata and the ability not to use the Volume as a whole. GDL also identifies and explores a wide range of parallelization strategies using simpler neural networks compared to traditional deep learning, leading to speed and memory efficiency [12, 13].
This thesis aims to create a GDL model to automatically detect LVOs, by analyzing the topological structure using distance features to classify them into LVO left, right or no LVO. This approach will be directly
compared to a state-of-the-art LVO CNN model, like in Thamm et al [9]. In summary, the thesis deals with the following points:
- Preparation of distance features for vessel graph
- Training of GDL models to analyze the graph topology
- Implementation of vessel-tree specific augmentation techniques
- Analysis and evaluation of the GDL models
References
[1] Walter Johnson, Oyere Onuma, Mayowa Owolabi, and Sonal Sachdev. Stroke: a global response is needed.
Bulletin of the World Health Organization, 94(9):634–634A, 2016.
[2] Geoffrey A. Donnan, Marc Fisher, Malcolm Macleod, and Stephen M. Davis. Stroke. The Lancet,
371(9624):1612–1623, 2008.
[3] Konark Malhotra, Jeffrey Gornbein, and Jeffrey L. Saver. Ischemic Strokes Due to Large-Vessel Occlusions
Contribute Disproportionately to Stroke-Related Dependence and Death: A Review. Frontiers in neurology,
8:1–5, 2017.
[4] Nikita Lakomkin, Mandip Dhamoon, Kirsten Carroll, Inder Paul Singh, Stanley Tuhrim, Joyce Lee, Johanna T. Fifi, and J. Mocco. Prevalence of large vessel occlusion in patients presenting with acute ischemic
stroke: a 10-year systematic review of the literature. Journal of neurointerventional surgery, 11(3):241–245,
2019.
[5] Murugan Palaniswami and Bernard Yan. Mechanical Thrombectomy Is Now the Gold Standard for Acute
Ischemic Stroke: Implications for Routine Clinical Practice. Interventional neurology, 4(1-2):18–29, 2015.
[6] Salwa El Tawil and Ketih W Muir. Thrombolysis and thrombectomy for acute ischaemic stroke. Royal
College of Physicians, pages 161–165, 2017.
[7] M. D. Ralf W. Baumgartner and et al. Transcranial color–coded duplex sonography, magnetic resonance
angiography, and computed tomography angiography: Methods, applications, advantages, and limitations.
Journal of Clinical Ultrasound, pages 89–111, 2005.
[8] M. D. Hidehisa Nishi, Naoya Oishi, MD, PhD, Akira Ishii, MD, PhD, and et al. Predicting Clinical
Outcomes of Large Vessel Occlusion Before Mechanical Thrombectomy Using Machine Learning. American
Heart Association Journals, pages 2379–2388, 2019.
[9] Florian Thamm, Oliver Taubmann, Markus J¨urgens, Hendrik Ditt, and Andreas Maier. Detection of Large
Vessel Occlusions using Deep Learning by Deforming Vessel Tree Segmentations. EESS.IV, pages 1–7,
2021.
[10] Lucas W. Remedios, Sneha Lingam, Samuel W. Remedios, Riqiang Gao, Stephen W. Clark, Larry T.
Davis, and Bennett A. Landman. Comparison of convolutional neural networks for detecting large vessel
occlusion on computed tomography angiography. Medical physics, 48(10):6060–6068, 2021.
[11] Stavros Georgousis, Michael P. Kenning, and Xianghua Xie. Graph Deep Learning: State of the Art and
Challenges. IEEE Access, 9:22106–22140, 2021.
[12] Si Zhang, Hanghang Tong, Jiejun Xu, and Ross Maciejewski. Graph convolutional networks: a comprehensive review. Computational Social Networks, 6(1):626, 2019.
[13] Federico Monti, Davide Boscaini, and et al. Geometric Deep Learning on Graphs and Manifolds Using
Mixture Model CNNs. IEEE Xplore, (2017):5115–5124.